New, Detailed Snapshots Capture Photosynthesis at Room Temperature
New X-ray methods have captured the highest resolution room-temperature images of photosystem II.
Menlo Park, Calif. — The living machinery responsible for photosynthesis – while commonplace and essential to life on Earth – is still not fully understood. One of its molecular mysteries involves how a protein complex, photosystem II, harvests energy from sunlight and uses it to split water into hydrogen and oxygen. This process generates the oxygen in the air that we all breathe.
New X-ray methods at the Department of Energy’s SLAC National Accelerator Laboratory have captured the highest resolution room-temperature images of this protein complex, which allows scientists to closely watch how water is split during photosynthesis at the temperature at which it occurs naturally. The research team took diffraction images using the bright, fast pulses of X-rays at SLAC’s X-ray free-electron laser – the Linac Coherent Light Source (LCLS), a DOE Office of Science User Facility. Nature published the study on Nov. 21.

Details of Photosynthesis Captured by SLAC’s X-ray Laser
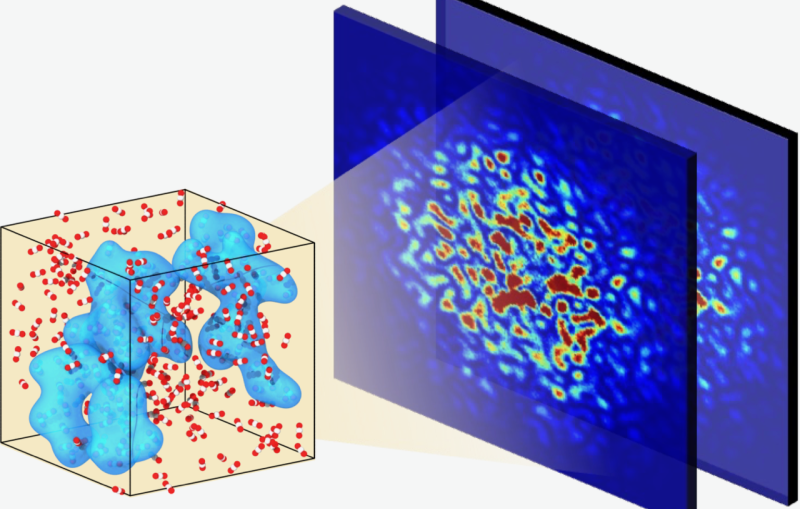
New X-ray methods at SLAC have captured the first detailed images showing how water is split during photosynthesis at the temperature at which it occurs naturally. The research team took the images using the bright, fast pulses of light at SLAC’s X-ray free-electron laser – the Linac Coherent Light Source (LCLS), a DOE Office of Science User Facility.
Chris Smith/SLAC National Accelerator Laboratory
Previously, the resting state of photosystem II had been seen in detail using samples that were frozen. In this latest study, the researchers were able to see two key steps in photosynthetic water splitting under conditions as it occurs in nature, a big step to decoding how the process works in detail. A damage-free, room temperature study means there are fewer artifacts in the images, and this gives a clearer picture of how photosystem II works in nature.
For many years now, scientists have been trying to understand this process in meticulous detail. Besides its fundamental importance to science, understanding water splitting might help develop ways to create artificial photosynthesis devices that can serve as potential clean energy sources.
“The eventual goal is to emulate what photosynthesis has been doing for about three billion years. This has been a research challenge for decades,” said Junko Yano, principal investigator and senior scientist at Lawrence Berkeley National Laboratory. “We now have the right tool, the femtosecond X-ray laser pulses created at LCLS, that allows us to observe the water-splitting reaction as it happens, in real time, and as it happens in nature.”
“We want to have enough snapshots of this process to see how exactly the oxygen is formed,” added Uwe Bergmann, a distinguished scientist at SLAC, and co-author of the Nature paper. “This method – to be able to get high resolution images at room temperature and under illumination in real time – is really the strategy we need to catch the molecule in the act.”
The international research team is a long-standing collaboration between SLAC and Berkeley Lab, and includes Humboldt University in Germany, Umeå University and Uppsala University in Sweden, Stanford University, Brookhaven National Laboratory and University of Oxford in the United Kingdom.
New Techniques and New Research Directions
In previous high-resolution studies of the system at synchrotron light sources, samples experienced radiation damage from longer exposure to X-rays. At LCLS, the researchers were able to take advantage of the ultrafast laser pulses to collect X-ray crystallography and spectroscopy data before damage occurred.
"The beauty of the LCLS is that the laser pulses are so short – only 40 femtoseconds in duration, but very intense – that you can collect the data before the sample is destroyed,” said co-author Jan Kern, scientist at Berkeley Lab and SLAC, in a related press release. "It's very new, and there are only two places in the world, LCLS and the SPring-8 Angstrom Compact free electron LAser (SACLA), where this can be done at present."
In a recent experiment at SACLA, the only other operating hard X-ray free-electron laser, another team of scientists was able to look at the first step in the cycle in a frozen sample, and under dark conditions – which means the water-splitting reaction had not yet been initiated in the protein complex.
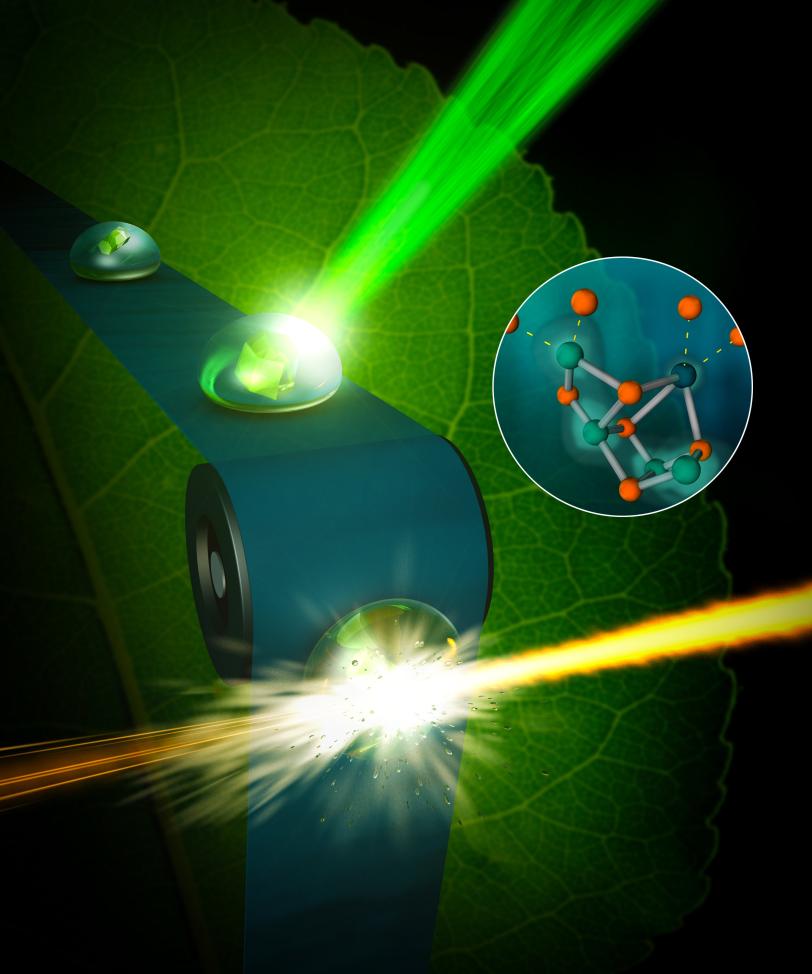
This latest study at SLAC catches this first step, as well as another step that is two stages further along in the four-step cycle, and at the same temperature the process normally occurs in nature. To do this, the scientists placed droplets of the sample in a solution with small, crystallized forms of the photosystem II on a moving conveyor belt and illuminated the samples with pulses of green light from a laser to initiate the water-splitting reaction. After two light pulses, they captured images of the crystals using X-rays, with a resolution finer than 2.5 angstroms – significantly better than previously achieved at room temperature.
Zeroing in on Water-splitting
The water-splitting reaction takes place at a metal catalyst within the photosystem II protein, known as the oxygen-evolving complex, that is made up of four manganese atoms and one calcium atom. The complex uses the energy from light to form pure oxygen from two water molecules. The four manganese atoms are critical in shuffling electrons through the cycle, but it is unknown where exactly in the complex the involved water is located or where the oxygen formation occurs.
To sort this out, the researchers used ammonia, a water substitute, to narrow down where oxygen atoms from two water molecules combine to form an oxygen molecule. If the ammonia was bound to a site, and the reaction still proceeded, that site is unlikely to be part of the oxygen molecule formation. The results from this study offered a surprise – the data do not seem to support two leading theories for how the reaction proceeds within the oxygen-evolving complex.

In future studies using this same technique, the researchers hope to capture more images at different steps of the process, which will allow them to further refine the details of the water-splitting reaction.
“The chemistry is so unusual,” co-principal investigator and senior scientist, Vittal Yachandra at Berkeley Lab, said “Learning how exactly this water-splitting process works will be a breakthrough in our understanding, and it can help in the development of solar fuels and renewable energy.”
SLAC scientists from the Stanford PULSE Institute and the Stanford Synchrotron Radiation Lightsource were also involved with this study. The research was supported by the DOE Office of Science and the National Institutes of Health, among other funding agencies. Some of the work for this experiment was conducted at the Advanced Light Source and the National Energy Research Scientific Computing Center, both DOE Office of Science User Facilities at Berkeley Lab.
Citation: Young et al., Nature, 21 November 2016 (10.1038/nature20161)
Press Office Contact: Manuel Gnida, mgnida@slac.stanford.edu, (650) 926-2632
About SLAC
SLAC National Accelerator Laboratory explores how the universe works at the biggest, smallest and fastest scales and invents powerful tools used by researchers around the globe. As world leaders in ultrafast science and bold explorers of the physics of the universe, we forge new ground in understanding our origins and building a healthier and more sustainable future. Our discovery and innovation help develop new materials and chemical processes and open unprecedented views of the cosmos and life’s most delicate machinery. Building on more than 60 years of visionary research, we help shape the future by advancing areas such as quantum technology, scientific computing and the development of next-generation accelerators.
SLAC is operated by Stanford University for the U.S. Department of Energy’s Office of Science. The Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time.